In which I answer your questions about cement
by Jason Crawford · October 31, 2019 · 8 min read
I recently turned an old post on cement into a surprisingly popular Twitter thread:
Someone asked me recently what's a boring topic that I'm excited/fascinated by.
— Jason Crawford (@jasoncrawford) October 17, 2019
That's easy: cement.
If it's wasn't thousands of years old, cement would seem futuristic. Think about it: it's *liquid rock*!
Here's how it works:
Many people replied with questions that I couldn’t answer to my satisfaction, such as:
- What was it about the volcanic ash the Romans added to their cement that made it set underwater? How does that work?
- Why was Portland cement stronger than previous cements?
- If cement re-absorbs CO2 from the air, the lime cycle would seem to be carbon-neutral; why is cement cited as a major CO2 contributor?
- Did the Romans have a lost formula for cement that we’ve been unable to surpass?
So I went back for another round of research. Here’s what I found.
First, a mea culpa: my original post and Twitter thread may have been misleading about the chemistry of cement, because I myself was not clear on it. The “lime cycle” that I described is correct—but it only applies to the most ancient, primitive form of cement (which may not even be strong enough for a practical concrete). The cement used by the Romans, as well as pretty much all cement used since the Industrial Revolution, uses different chemistry.
To recap: Cement comes from limestone, which contains the mineral calcium carbonate (CaCO3). The stone is crushed and kilned, or “calcined”, which releases CO2 and leaves “quicklime”, calcium oxide (CaO).
In a pure lime cement, the quicklime is mixed with water, which forms “slaked lime”, or calcium hydroxide (Ca(OH)2). When this sets, it re-absorbs CO2 from the atmosphere and turns back into calcium carbonate, completing the lime cycle. Because of this, such a cement can’t be poured too thick (or the middle layers won’t be exposed to the air), and it doesn’t set underwater (where it can’t absorb carbon dioxide). Further, this type of cement apparently doesn’t weather well. (It’s only used today for things like restoring historic brickwork.)
However, in other varieties of cement, something very different happens. When lime is mixed with silicates—from volcanic ash or rock, or from clay—the chemistry is changed completely. When this form of cement is mixed with water, the water reacts with the lime and the silicates together to form calcium silicate hydrates. This cement then sets and hardens without absorbing CO2, which is how it can happen underwater. Because of this property, this class of cements is called “hydraulic cement”.
How does hydraulic cement set and harden? Apparently we still don’t completely understand it! It’s an active area of research, and people get PhDs in cement chemistry. But it seems that the calcium silicate hydrate, or C-S-H (in cement chemist notation), forms crystals which stretch out in rods or tendrils. The tight interlocking of these crystal threads gives this cement its strength.
There are more details than this—other minerals are involved, including aluminates and ferrite—but they mostly affect the cement kilning process, and don’t seem to contribute to cement strength. A modern Portland cement is about two-thirds lime, one-fifth silica, and a small percent each of alumnia, ferrite, and other minerals.
Of course, the Romans didn’t know all of this. Vitruvius, a Roman engineer and architect from the 1st century BC, in his famous Ten Books on Architecture, simply wrote:
There is also a kind of powder from which natural causes produces astonishing results. This substance, when mixed with lime and rubble, not only lends strength to buildings of other kinds, but even when piers are constructed of it in the sea, they set hard under water.
That powder was volcanic ash from Mt. Vesuvius, found near the village of Pozzuoli and other sites; hence the term “pozzolana”.

After the fall of the Roman Empire, this formula and other Roman cement-making techniques were largely lost in Europe for a thousand years, to be rediscovered only during the Renaissance. A complete copy of Vitruvius’s On Architecture was discovered in a Swiss monastery in 1414, and in the early 1500s, a builder named Fra Giovanni Giocondo decided to try the ancient Roman techniques. He used pozzolana to make hydraulic cement to rebuild the Pont Notre-Dame in Paris, after the previous wooden bridge had collapsed.
Sometime in the 16th or 17th century, another additive like pozzolana was discovered: a different volcanic rock known as trass, found near the town of Andernach on the Rhine River in what is now Germany. Dutch traders began selling it in the 1600s, and it spread throughout Europe.
During the Industrial Revolution, engineers and inventors began to experiment more systematically with cement recipes, and discover new techniques.
John Smeaton, called the father of civil engineering, was tasked in the 1750s with rebuilding the Eddystone Lighthouse. The first few lighthouses at its spot had fallen, and Smeaton wanted his structure to last. Wood would not do—especially since the previous lighthouse had burned down. Stone was required. But the rock it sat on was submerged at high tide, so the mortar had to withstand the constant battering of the sea.

To find the best cement for the job, Smeaton systematically tested lime from multiple sources, with and without additives such as pozzolana and trass. He found that lime from stone quarried near the small town of Aberthaw in Wales made a good hydraulic cement, even without additives. This stone, it turned out, was naturally mixed with clay, and the clay provided the silicates that were normally supplied by volcanic rock. (He ultimately settled on lime made from this stone, combined with additional pozzolana, which is the formula that won his trials.)
The Eddystone Lighthouse stood for over a hundred years, and was retired in 1876 only when the rock underneath had eroded too much for it to stand. The lighthouse itself was still solid—it had been built stronger than its foundation.
The cement Smeaton had discovered, from limestone deposits naturally mixed with clay, came to be known as “natural cement”. Smeaton published a book about the building of the lighthouse, including his cement experiments, in 1791 (over 30 years after it was completed; I’m not sure why the delay). After that, several varieties of natural cements were manufactured in Britain, some of which was marketed as “Roman cement” (a confusing term, since the Romans actually used pozzolanic cement, not natural cement).
Then, in the 1840s, a new recipe was discovered that would come to dominate the entire field, up to the present day.
Briefly, if the right mixture of lime and silicates is heated to a high enough temperature, it forms very hard nodules, called “clinker”. Clinker was probably created by accident occasionally by previous cement makers, when they “overcooked” their lime, but the nodules are extremely difficult to grind, so it was considered a botched product. When clinker is ground up, however, the resulting cement is stronger than any other formula, including pozzolanic or natural cement. When it was first invented, it was tested and found to be almost twice as strong as the best competitor.

This basic formula is now known as Portland cement (after the town in England, not the one in Oregon or Maine), and variations on it make up virtually all cement in use today. The history is murky, but it seems to have been discovered by William Aspdin around 1841, and then reverse-engineered by Isaac Charles Johnson a few years later. (To make things even more confusing, the first cement to be marketed as “Portland cement” was made earlier by William’s father, Joseph Aspdin; however, that cement was a natural cement, and would not today be called a Portland cement.)
One of the advantages of Portland cement is its “early strength.” Cement, after being poured, goes through two phases: setting, followed by hardening. Setting is the process in which the liquid cement becomes stiff or rigid, and normally happens in a matter of hours. At this point, however, the concrete is not at its full strength. Hardening is the process by which concrete slowly gains strength over time, a matter of weeks or longer. In modern industrial usage, “early strength” is measured at 7 days, and concrete is nominally at “full strength” after 28 days. Rapid strength gain is important in construction; the faster concrete hardens, the faster you can build. With the high early strength of Portland cement, the formwork holding concrete in place can be removed in a matter of days.
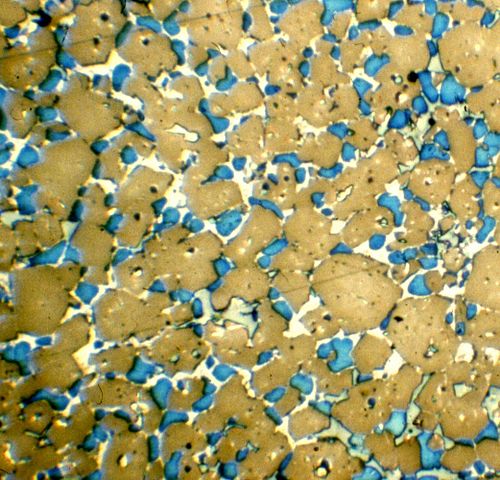
Portland cement was an accidental discovery, but today we understand something about the chemistry. Lime and clay, when kilned together, form calcium silicate compounds. The most common phase is “belite”, or dicalcium silicate (2CaO · SiO2, or C2S in cement chemist notation). However, over about 1300° C (about 2400° F), a new phase forms, “alite”, or tricalcium silicate (3CaO · SiO2, or C3S). Alite gives Portland cement its strength, especially early strength.
So did the ancient Romans have a special recipe for cement that we haven’t been able to surpass? The short answer is no. The longer answer is maybe, in certain contexts.
It’s true that the exact formula used by the Romans has been lost, like so many things from that era, and we don’t exactly know how to duplicate it. But that doesn’t mean that Roman concrete was better than modern. Portland cement makes for a stronger concrete than anything the Romans used, and we have much more precise control over its manufacture, with modern chemical testing and computer-controlled plants.
Further confounding the issue is that Roman structures do last much longer than those built using modern techniques. In particular, reinforced concrete, with steel “rebar” or other structures, is subject to corrosion and breakdown of the steel itself, giving the concrete a lifetime of only about 50–100 years. Unreinforced concrete can last for thousands of years, as in the famous Pantheon in Rome. This is a deliberate tradeoff we make today in order to get the huge advantages of reinforced concrete, namely tensile strength. This allows us to make many more structures, including modern skyscrapers, not just the arches and domes of ancient Roman architecture.

(Incidentally, it seems that the Romans experimented with reinforced concrete, but failed because they used bronze, which has a different coefficient of expansion with heat vs. concrete. Iron’s coefficient is similar enough that it works! Why the Romans didn’t try iron reinforcement, I’m not sure.)
There does seem to be one particular application in which the Romans had a formula we would like to rediscover: maritime concrete, exposed to seawater. Normally, concrete in a pier or harbor erodes over time, both due to the salts and other minerals in the seawater, and due to the abrasion of sand and silt. However, some Roman marine structures have survived for 2,000 years. Geologists studying Roman concrete have recently discovered (2017) that the particular concrete formula used in those structures has a beneficial interaction with seawater, creating new crystals within the cementing matrix that actually increase strength. DARPA just announced that “Unlocking the Secrets of Roman Concrete” is one of many topics for exploration under a research awards program.

In general, be suspicious of sensational headlines about ancient mysteries and lost secrets. It’s faddish today to think that our distant ancestors were smarter or wiser than we are, or that old traditions somehow outperform the latest technology, but that’s almost never true. It’s long past time to discard ancestor-worship and to embrace the idea of progress.
Sources and further reading
Concrete Planet, by Robert Courland
The excellent sites Understanding Cement, written by Nick Winter of WHD Microanalysis Consultants; and Cement Kilns (which is about much more than just the kilns), written by Dylan Moore, who also very kindly wrote a detailed email reply to some of my questions about cement manufacturing
Encyclopedia Britannica on “History of cement”, and many pages on Wikipedia including “Cement” and “Portland cement”
